Organic Molecules worksheet Organizational Chart for Biomolecules
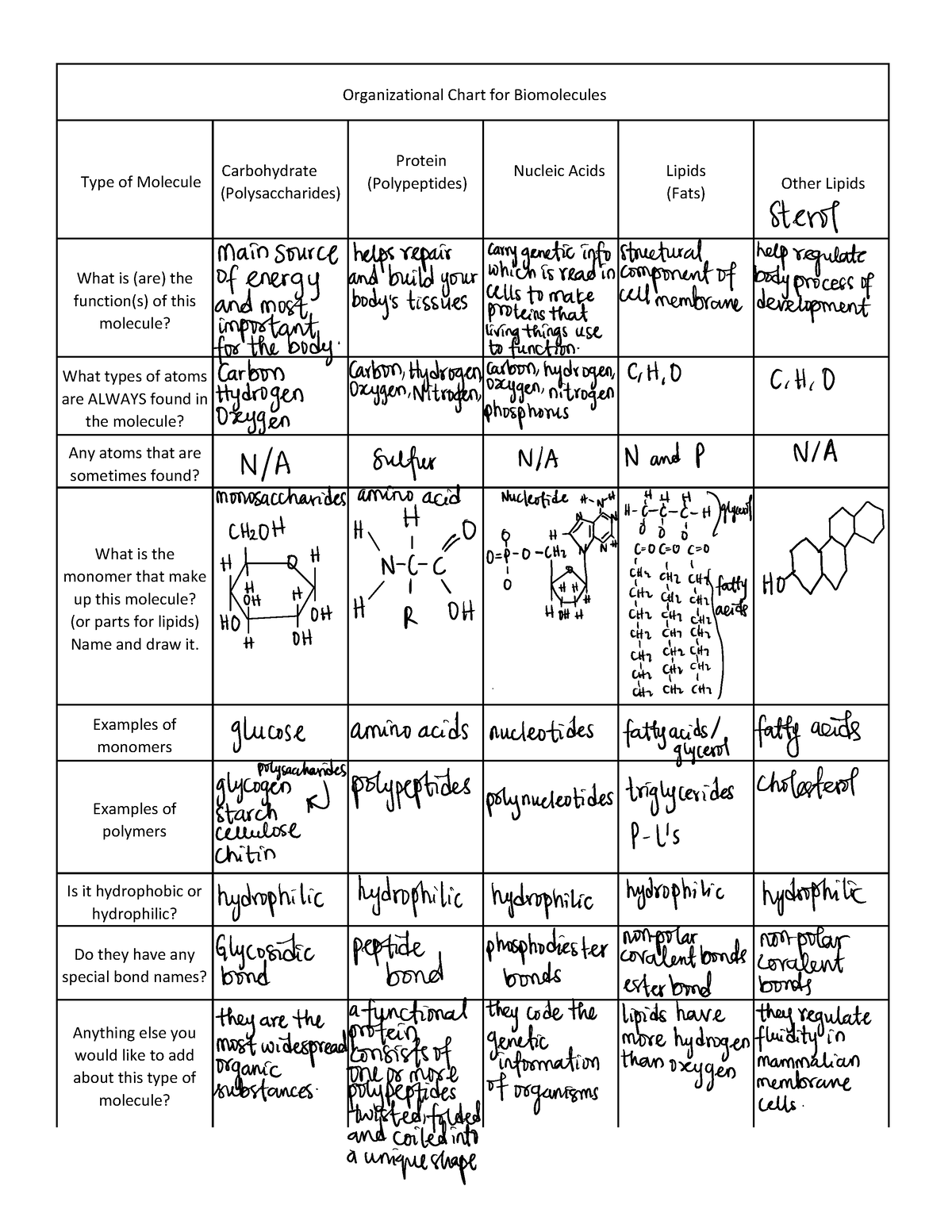
Organic Molecules worksheet Organizational Chart for Biomolecules
Introduction. In its simplest definition, organic compounds include all molecules that contain carbon. By this definition, simple molecules such as carbon monoxide (CO) and carbon dioxide (CO2) would be defined as organic molecules, however, these simple molecules behave more like inorganic molecules than organic molecules.

Organic Molecules Chart Organic Molecules Contrast Chart school
Tim Soderberg. University of Minnesota Morris. An understanding of the various types of noncovalent forces allows us to explain, on a molecular level, many observable physical properties of organic compounds. In this section, we will concentrate on solubility (especially solubility in water), melting point, and boiling point.
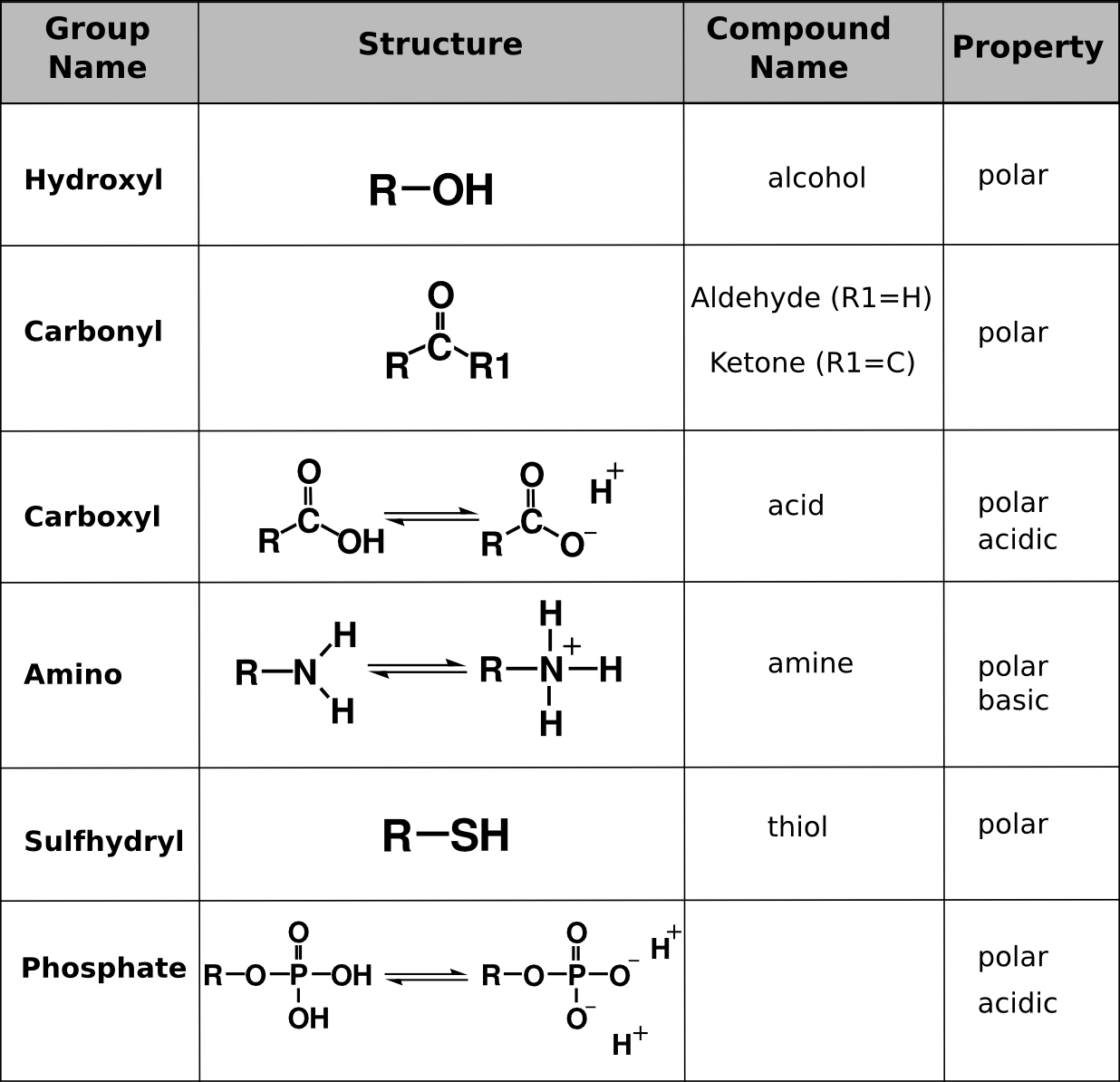
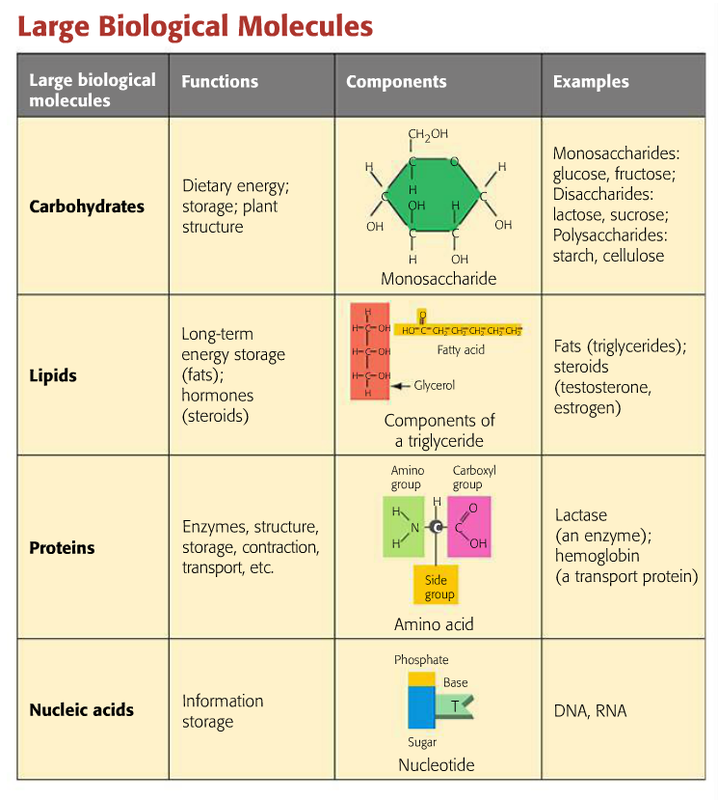
2.3 Biologically Important Macromolecules Biology LibreTexts
Structure search. Search by Structure or Substructure. Upload a structure file or draw using a molecule editor.
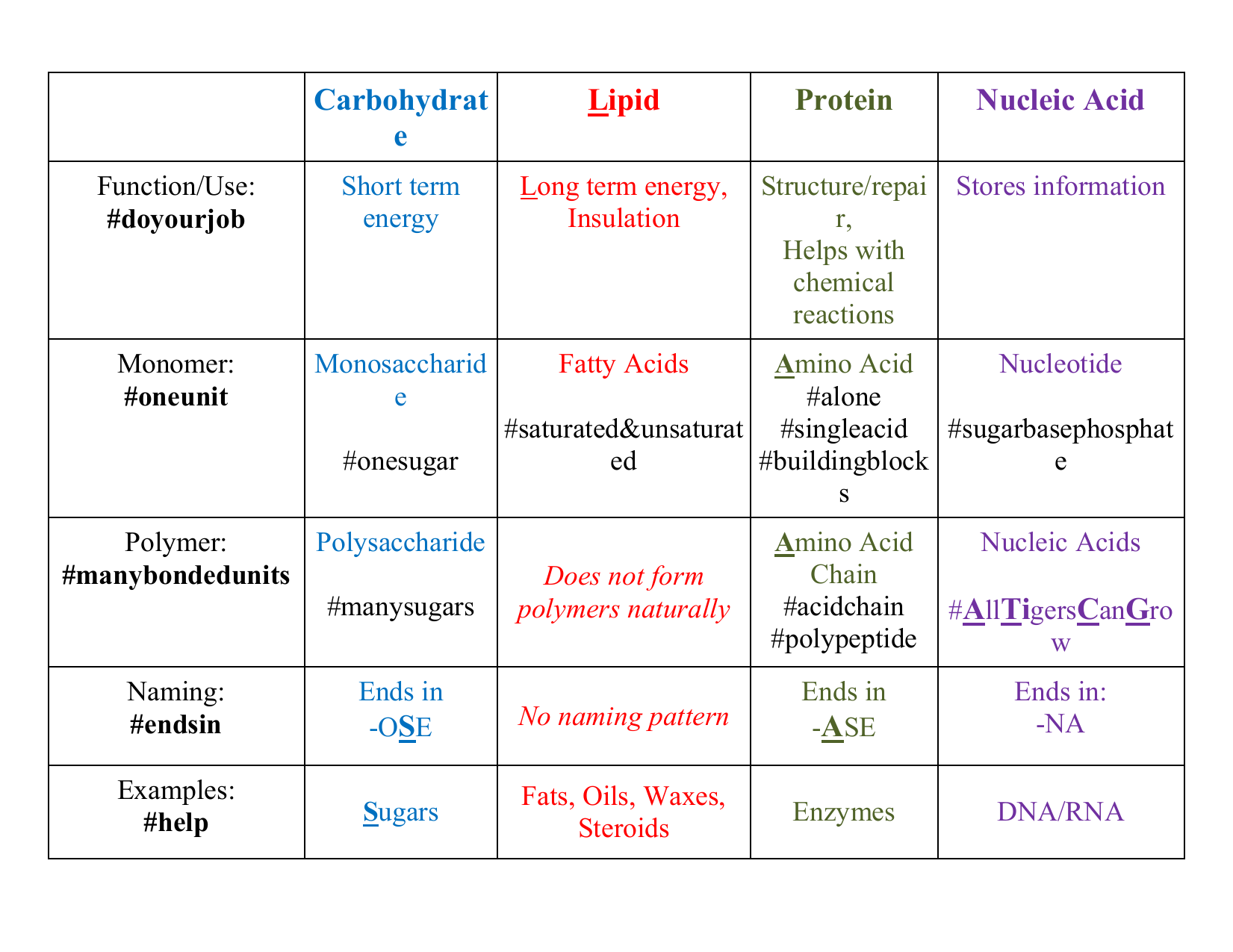
Organic Molecule Chart
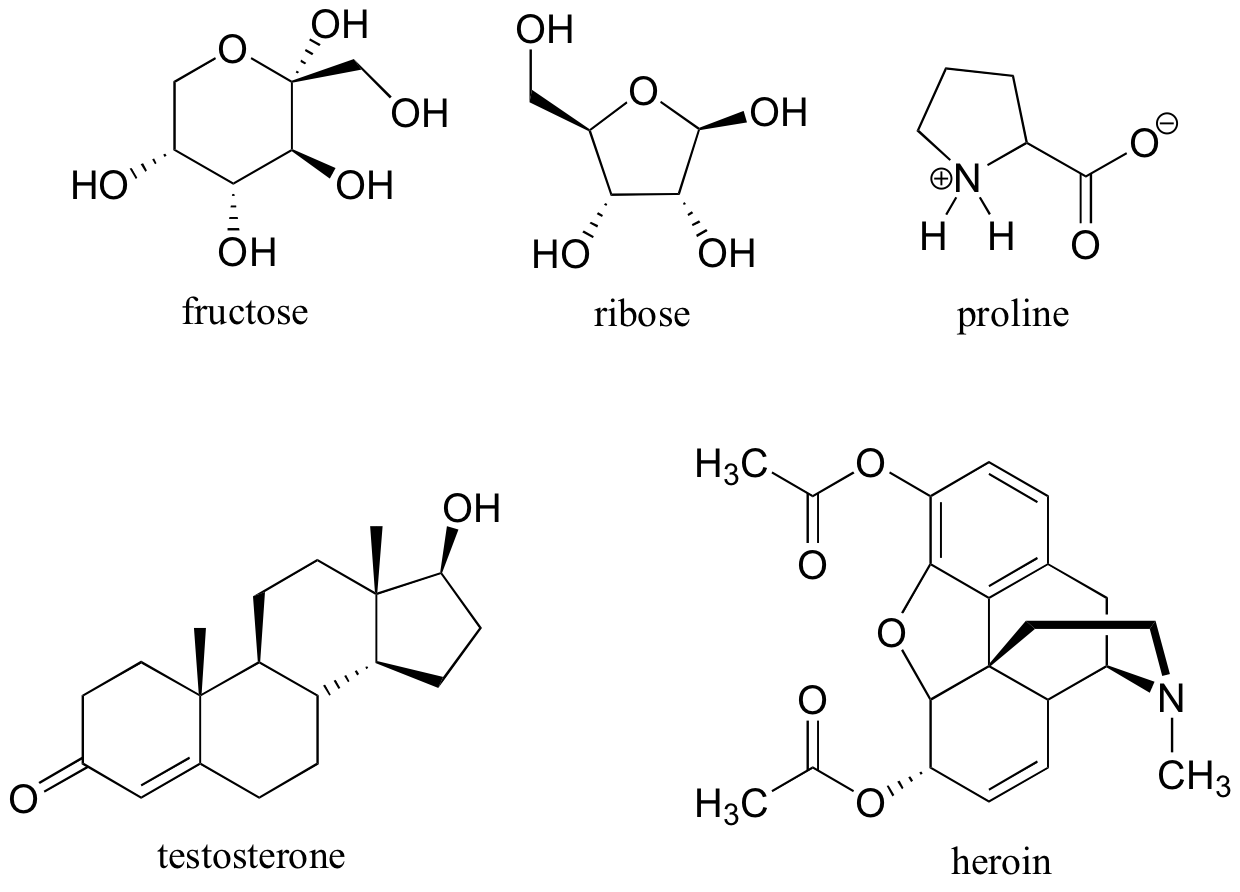
Carbohydrates are the first class of organic molecules. The simplest kind of carbohydrate includes monosaccharide simple sugars, which have a basic formula: two hydrogens and one oxygen for every carbon atom or one water for every carbon. Glucose is a common carbohydrate whose formula is C6H12O6. Sugars can be either a single sugar molecule to.
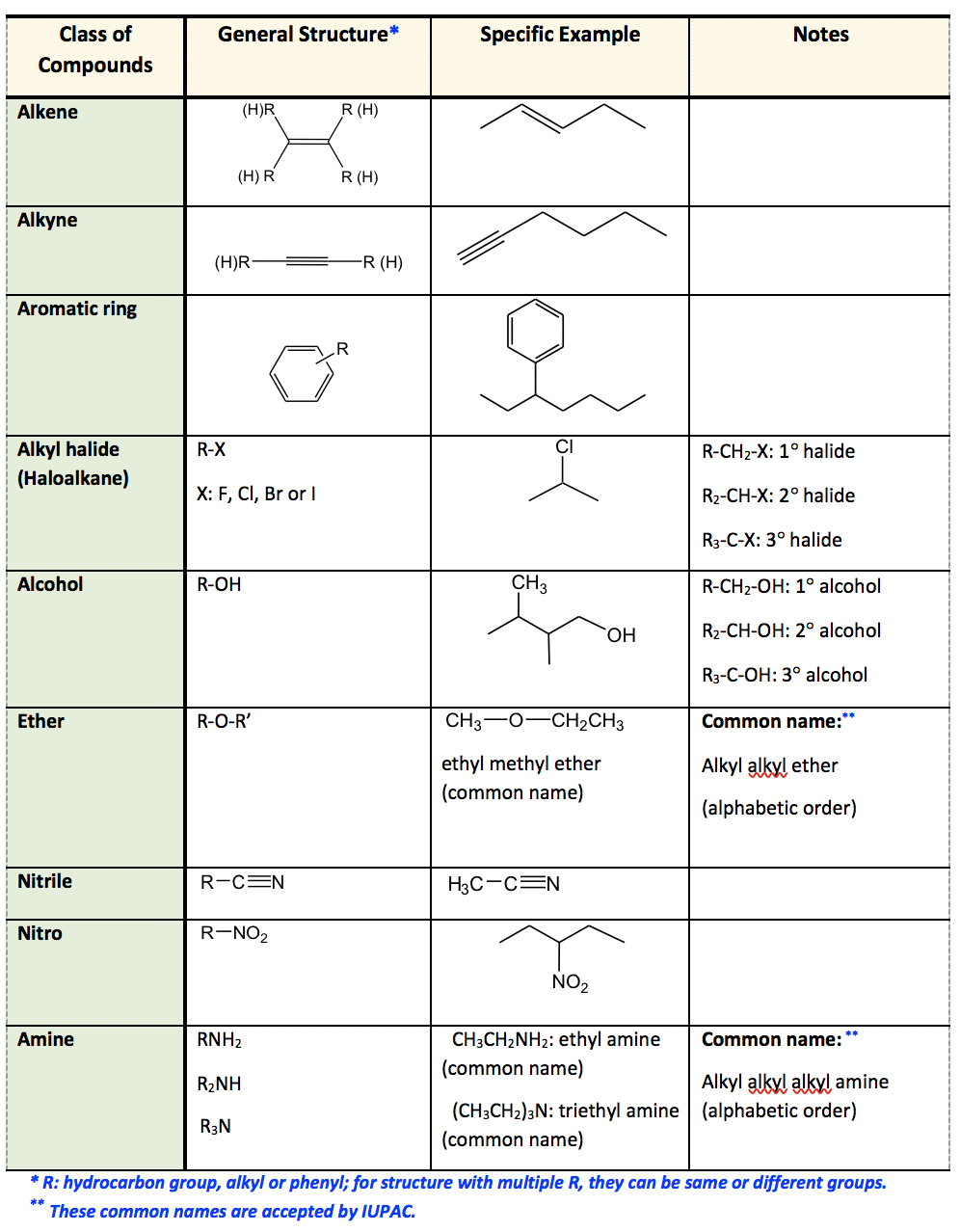
2.3 Functional Groups Organic Chemistry I
The chemistry of these compounds is called organic chemistry. Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds.
3.2 Conformations of cyclic organic molecules Chemistry LibreTexts
A further complication is that, even outside of a biological context, many simple organic molecules are known almost universally by their 'common', rather than IUPAC names. The compounds acetic acid, chloroform, and acetone are only a few examples. In biochemistry, nonsystematic names (like 'cocaine', 'capsaicin', 'pyruvate' or.
Organic Molecules Chart
For many purposes, ball-and-stick models of organic compounds give useful information about the spatial relationships of the atoms, and for \(CX_4\) the angles between sticks are set at \(109.5^\text{o}\) (Figure 2-1). Organic molecules strongly resist deformation forces that alter their valence angles from normal values.

Organic Molecules Chart
Meaning. A large, organic molecule such as carbohydrates, lipids, proteins, and nucleic acids. A molecule that is a building block for larger molecules (polymers). For example, an amino acid acts as the building blocks for proteins. A large molecule made of repeating subunits (monomers).
Organic Molecules Mrs. CovarrubiasAdvanced Biology
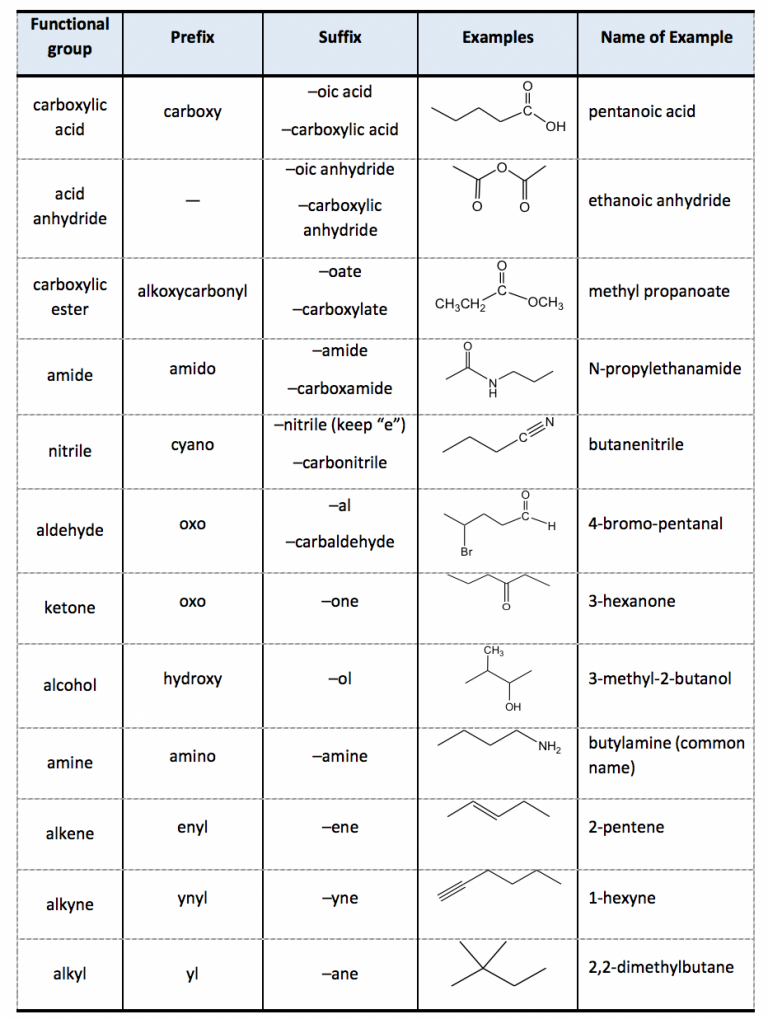
The purpose of this chart will be clear if you've got a background in chemistry. If you haven't, it's a useful tool to decode the different parts that make up molecules in organic chemistry. All carbon-based (organic) molecules contain functional groups - some more than one of them - and they're what gives molecules their particular.

The table shows the energy that is stored in three types of organic
Drawing the Structure of Organic Molecules. Although larger molecules may look complicated, they can be easily understood by breaking them down and looking at their smaller components. All atoms want to have their valence shell full, a "closed shell." Hydrogen wants to have 2 e - whereas carbon, oxygen, and nitrogen want to have 8 e -.

Organic functional groups chart expanded edition M A N O X B L O G
1. Organic compounds containing substituents from Group C are named following this sequence of steps, as indicated on the examples below: •Step 1. Find the longest continuous carbon chain. Determine the root name for this parent chain. In cyclic compounds, the ring is usually considered the parent chain, unless it is
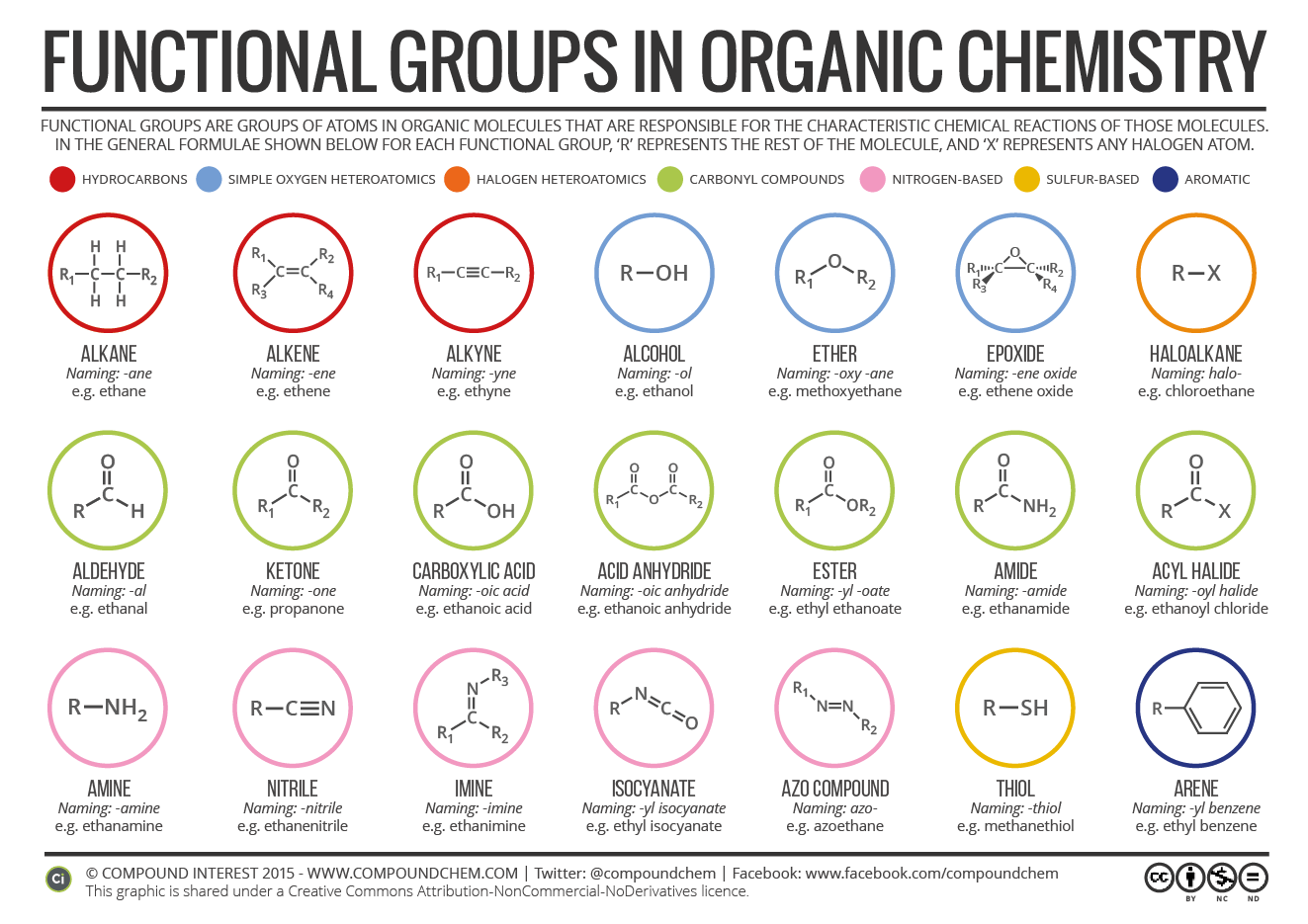
Functional Groups in Organic Compounds
They are small, simple compounds that play important roles in the cell, although they do not form cell structures. Most of the carbon found in organic molecules originates from inorganic carbon sources such as carbon dioxide captured via carbon fixation by microorganisms. Exercise 7.1.2 7.1. 2. Describe the most abundant elements in nature.

😂 Which is an organic molecule. CHEMISTRY II WATER AND ORGANIC
The structures, abbreviations (both three- and one-letter), and pK a values of the 20 amino acids commonly found in proteins are shown in Table 26.1.All are α-amino acids, meaning that the amino group in each is a substituent on the α carbon—the one next to the carbonyl group. Nineteen of the twenty amino acids are primary amines, RNH 2, and differ only in the nature of their side chain.

Chemical structure and common names of the 16 organic compounds used in
Figure 26.1. 2: The Tetrahedral Methane Molecule. Methane (CH 4 ), ethane (C 2 H 6 ), and propane (C 3 H 8) are the beginning of a series of compounds in which any two members in a sequence differ by one carbon atom and two hydrogen atoms—namely, a CH 2 unit. The first 10 members of this series are given in Table 26.1.

chemistry nomenclature Organic chemistry, Chemistry education
Table 2.4 Subordinate Groups. We will go through several examples for more details about the naming rules. 1. The parent structure is the 6-carbon carboxylic acid with a double bond, so the last name comes from "hexene". To add the suffix, the last letter "e" will be dropped, so the parent name is "hexeneoicacid".

Probable chemical mechanisms of different classes of organic compounds
Organic chemistry 14 units. Unit 1 Structure and bonding. Unit 2 Resonance and acid-base chemistry. Unit 3 Alkanes, cycloalkanes, and functional groups. Unit 4 Stereochemistry. Unit 5 Substitution and elimination reactions. Unit 6 Alkenes and alkynes. Unit 7 Alcohols, ethers, epoxides, sulfides. Unit 8 Conjugated systems and pericyclic reactions.